Glutathione
Glutathione (GSH) - Your Whey to Health
Milk, Glutathione, & Whey Protein We should not find it surprising that milk contains so many life sustaining and life protecting components. Milk is provided to the newborn in all mammals as a tailor-made food, containing bioactive components that strengthen the newborn's naive immune system, components to insure healthy intestinal flora, and of course nutritional components perfect for growth and development. The challenge has been to isolate and stabilize the fragile bioactive components of milk.... one of which is Whey Protein. Whey protein concentrates are of particular interest to the practitioner due to their wide-range and near full-blend of essential and non-essential amino acids, which are commonly referred to as the building blocks of life. Linked amino acids combine to form proteins. These protein comprise nearly every tissue and organ in the body, therefore any supplementation of the diet with proteins may be beneficial to injury repair, metabolism, and general health. |
|
What is Glutathione exactly?
Glutathione (GSH) is a small protein molecule formed from the amino acids cysteine, glycine, and glutamic acid. Glutathione is manufactured inside your cells. Your cell's ability to make glutathione is directly determined by the supply of raw materials, or
precursors. In particular, the amino acid cysteine.
Glutathione is the Master Antioxidant
Glutathione is your body's master antioxidant and one of the most important cleansing and healing agents.
Glutathione blocks free radical damage and helps to recycle Vitamins E and C, therefore plays a key role in their function. Because
Glutathione exists within the cells, it is in a prime position to neutralize free radicals. The highest concentration of glutathione is found in the liver which is the principal organ involved in the detoxification and elimination of toxic materials.
Glutathione may also help with detoxification by binding to toxins such as heavy metals, solvents and pesticides, allowing them to be excreted in urine or bile.
Glutathione is a "Superfood"
Glutathione is one of the 14 Superfoods listed in "SuperFoods Rx : Fourteen Foods That Will Change Your Life", co-authored by Dr. S. Pratt, an authority on food and aging.
The list is based on an exhaustive study of the scientific research behind the world's best daily diets, and includes the 14 nutrients that show up consistently in the most health-promoting, disease-preventing, anti-aging diets in the world.
Glutathione is the "Life Extension Molecule"
Low glutathione levels are found in immune comprodegenerative diseases such as cancer, multiple sclerosis, AIDS, ALS, Alzheimer's, and individuals with neuro-Parkinson's disease, atherosclerosis, male infertility, pregnancy complications, cataracts.
High levels of glutathione appear to protect against the dangers of cancer, heart disease, premature aging, autoimmune diseases, damage from many pharmaceutical drugs, and chronic illnesses.
What the Experts say about Glutathione
|
"Glutathione is a substance, the levels of which in our cells are predictive of how long we will live. There are very few other factors which are as predictive of our life expectancy as is our level of cellular glutathione. Glutathione has been called the "master antioxidant", and regulates the actions of lesser antioxidants such as vitamin C, and vitamin E within the body.
"We literally cannot survive without this antioxidant."
Earl Mindell, R.Ph., Ph.D, in What You Should Know about the Super Antioxidant Miracle
"Without glutathione, other important antioxidants such as vitamins C and E cannot do their job adequately to protect your body against disease." Allan Somersall, Ph.D., M.D., and Gustavo Bounous, M.D. FRCS(C) in Breakthrough in Cell Defense
"No other antioxidant is as important to overall health as glutathione.
It is the regulator and regenerator of immune cells and the most valuable detoxifying agent in the human body. Low levels are associated with hepatic dysfunction, immune dysfunction, cardiac disease, premature aging, and death."
Lorna R. Vanderhaeghe & Patrick J.D. Bouic, Ph.D. in The Immune System Cure
"If there is one survival tool every HIV(+) person should consider, it is taking dietary supplements that increase glutathione production."
- Michael Mooney, Author of Built to Survive: A Comprehensive Guide to the Medical Use of Anabolic Steroids, Nutrition and Exercise for HIV (+) men and women
"Raising glutathione levels is a good thing, no matter how you do it. There are several theories about how to do this and all are slightly different. Some believe that NAC will do it, it certainly has the right chemical structure. Others think that only bioavailable glutathione is the way to go.... However you elevate your glutathione levels, it is a good thing to do!" -
Douglas T. Dieterich, M.D. in a response to a patient query at a forum on TheBody.com, an AIDS and HIV Information Resource.
One reason why it is so important to maintain high levels of
glutathione is that it is crucial for the detoxification of carcinogens. Packer states that most people do not inherit "cancer genes"; rather, they have a genetic weakness in their detoxification system. Glutathione is an extremely important part of the detoxification system, and thus of our defenses against cancer. Interestingly, whey protein has also been found to raise glutathione levels. Glutathione may also be one of the most important keys to longevity. Centenarians have been found to have higher levels of glutathione than would be expected for their age. Boosting one's glutathione levels ...should be one of the first items on anyone's anti-aging agenda.
- Ivy Greenwell in The Antioxidant Network, A brief review of "The Antioxidant Miracle," by Lester Packer, PhD and Carol Colman
"As Dr. Lombard points out in his book, "The Brain Wellness Plan"...the brainís high fat content renders it especially vulnerable to free radicals, so that the body has defined specific ways to protect brain cell fatty acids through special antioxidants....
Glutathione is one of the most powerful antioxidants in the body. Depressed glutathione levels are associated with the increased generation of free radicals found in Parkinsonís patients, for example, and contributes to further brain cell death." - Excerpts from the Willner Window Radio Show aired in May 98
A deficiency of glutathione can cause hemolysis (destruction of red blood cells, leading to anemia) and oxidative stress.
Glutathione is essential in intermediary metabolism as a donor of sulfhydryl groups which are essential for the detoxification of acetaminophen.
[PDR Medical Dictionary. Spraycar. 1999] Selenium is a structural component of, and a co-factor for the antioxidant enzyme lutathione peroxidase.
"(Glutathione peroxidases) break down hydrogen peroxide and any peroxides which form on fats and oils within the body. The selenium contained in the enzymes acts as the reactive centre, carrying reactive electrons from the peroxide to the glutathione. It is the glutathione that is the antioxidant in the reaction, not the selenium as many health food companies would lead us to believe. Selenium by itself is a potent oxidant which can be very toxic if to much is taken." - Dr Steven Gieseg in Reducing Free Radicals - A Dietary Revolution, New Zealand Science Monthy, (1999) July, 6-8.
|
Antioxidants and Free Radicals
- Bruce Ames, Ph.D. at University of California, Berkeley estimates that
every single one of our trillions of cells suffers about
10,000 free radical hits daily!
(Ames, 1993)
We know that glutathione is the master antioxidant. We read and hear about antioxidants and free radicals them all the time, but just what are they? The simple answer is that
antioxidants are substances that protect our cells from oxidation (by free radicals).
Let's look at what free radicals are. Molecules are composed of atoms bonded together, via the sharing of electrons. Generally, atoms exist in pairs, with each electron of the pair having an opposite spin to the other. When a molecule is split, two things can happen. First, the electrons can stay together. When this happens, we say the molecule has ionized. For example, table salt, which is Sodium Chloride (NaCl), ionizes into two charged ions, specifically a sodium ion and a chloride ion. In this case, the Chloride received the electron pair, and the Sodium lost it. The other thing that can happen is that the electron pair is split. This leaves a
highly reactive atom (a free radical), seeking to steal an electron, which sets up a domino effect of electron stealing.
Antioxidants work by offering easy electron targets for free radicals.
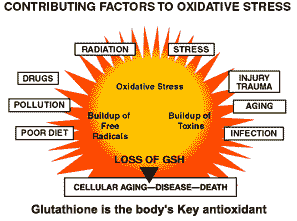
Each time you are confronted by the threats in the outer circle of the diagram free radicals are released. The natural reserve of glutathione in the body breaks down under the assault. The effects of oxidative stress are so critical in fact that a new field of medical science has emerged, called 'Free Radical Biology' which studies the long list of diseases caused by it. Today it is known that antioxidants help diminish cell damage, slows the harmful effects of aging and lessens the threat of disease. Oxidative stress can be minimized by raising intracellular GSH levels.
If the free radical is not neutralized by an antioxidant it can wreak all sorts of havoc in our bodies. Current research, for example, is pointing to the fact that it is not only the presence of fat in our bodies that leads to plaque formation on our arteries, but the oxidation of fat by free radicals. With cancer, the problem starts when free radicals chip away at the DNA of our cells, eventually causing mutations that lead to malignancies.
The antioxidants provided by vitamins work because they can donate electrons to free radicals, thus neutralizing them. However, this makes the vitamin molecule unstable, and now it needs an electron. This is sort of like passing a very hot potato
from person to person until someone finds a place to lay it down. In our bodies, that place is glutathione.
The chemical equation gets fairly technical, but in the end
glutathione donates an electron and becomes a stable molecule known as GSH. Glutathione can then be regenerated via an enzymatic pathway that involves lipoic acid. The details of the chemistry involved is beyond the scope of this page, but is well established in the scientific literature. Also well established is the fact that the glutathione antioxidant system is the most important system in our bodies when it comes to the destruction of reactive oxygen compounds (very potent free radicals).
(Meister, 1994)
Glutathione is often referred to as GSH.
Glutathione
In order to understand the mechanisms beneficial biochemical interactions and possible whey protein potential health benefits of WPC, a brief biochemical review of the protein Glutathione is required, since it is this protein more than any other that has been thought to provide a protective function for a number of organ systems, including the crystalline lens of the eye, the retina, prostate gland, and the immune system.
a. Glutathione Synthesis
For the body to produce Glutathione (GSH) six building blocks are required: L-glutamate, L-cysteine, L-glycine, magnesium, potassium, and 5' ATP. Two enzymes are also required and they are L-gamma-glutamyl-cysteine synthetase (equation 1) and glutathione synthetase (equation 2) and the reaction proceeds in the following manner:
Mg 2+
(1) L-glutamate + L-cysteine + ATP--------->
L-gamma -glutamylcysteine + ADP + P
Mg2+
(2) L-gamma-glutamylcysteine + L-glycine +ATP----------->
K+
L-gamma-glutamylcysteinylglycine +ADP + P
L-cysteine is the rate limiting substrate in this reaction12 , while the rate controlling enzyme for the reaction is L-gamma -glutamylcysteine synthetase(5).
b. Scavenger Pathways
The 2 major functions of glutathione are to detoxify hydrogen peroxide (H2O2) and other organoperoxidases (free radicals) and to defend against oxidation within cells via the Glutathione Redux Cycle, or more commonly referred to as the Scavenger Pathways(6,7).
Glutathione plays it's role of "scavenger" through out the body. The role of scavenger is primarily accomplished through glutathione peroxidase (GSH px). The peroxidase interacts with the H2O(2) to reduce it to harmless water, thus limiting it's electron stealing capacity. This is illustrated in the following equation:
(2) 2-glutathione-SH +ROOH ------>
glutathione disulfide + ROH + H2O
The disulfide is then reduced with the co-enzyme NADPH in the prescene of the enzyme Glutathione Reductase to yield the original glutathione compound.
(3) glutathione reductase +NADPH + H+ ----------->
2-glutathione-SH + NADP -
Many theories of aging and disease are based upon the interaction of the formation of free-radicals and the subsequent reduction in glutathione levels which allows for an accumulation of free-radicals to remain within a cell and organ or organ system. Free-radicals that remain within cells may cause cell damage, DNA damage and may even cause cell death, cancer transformation or loss of cell immunity to viral or bacterial infection.
|
GSH's metabolic functions include:
| ♦ Enhancement of Immune Function |
| ♦
Elimination of Toxins
|
| ♦ Elimination of Carcinogens |
| ♦ Antioxidant Cell Protection
|
| ♦ Protection against Ionizing Radiation
|
| ♦ DNA Synthesis and Repair |
| ♦ Protein Synthesis
|
| ♦ Prostaglandin Synthesis
|
| ♦ Leukotriene Synthesis
|
| ♦ Amino Acid Transport
|
| ♦ Enzyme Activity and Regulation
|
Where do we get these antioxidants?
Nature provides these through fresh
"whole" fruits and vegetables which are full of them. Asparagus, avocados and walnuts are particularly rich dietary sources of
Glutathione. (It is important to eat only
whole foods for optimal health.) Some important ones to look for are vitamins A, C, E, and Selenium. In the case of glutathione, the source is dairy in the form of
whey protein isolates.
High quality
nutritional supplements also provide effective antioxidants.
Cysteine Rich Whey Proteins, and their effect on Glutathione
Now that we've laid a foundation for the importance of glutathione, let's discuss the role of cysteine in glutathione synthesis. The importance of cysteine lies in the fact that it is an essential
precursor to the master antioxidant glutathione.
Glutathione (GSH) is a tripeptide (a very small protein) made up of three amino acids: L-glutamine (glutamatic acid), L-cysteine and L-glycine. The amino acid most important (known as the rate-limiting factor) is cysteine.
(Deleve, 1991)
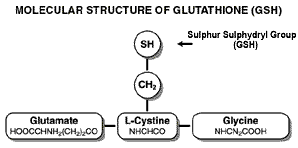
In order for cysteine to survive the trip from the mouth to your cells it must be a part of a larger protein. Without adequate cysteine, cells can't produce enough GSH and the body suffers on three levels: cellular oxidation contributes to general decline and aging, toxins accumulate in the body causing further damage, and the immune system is compromised, leaving the body vulnerable to disease.
However, there is a form of cysteine that is non-toxic, easily transported into the gut, and transferred into cells. This form is cysteine and is comprised of two cysteines joined together by a disulfide bond. Upon cell entry the cysteine is reduced to cysteine.
(Droege et al., 1994)
Having established that cysteine is the crucial element in supporting glutathione production, let's return to our cystine rich proteins. Alpha-lactalbumin, lactoferrin and serum albumin are all high in cysteine. Serum albumin and lactoferrin are also high in the combined molecule of glutamine and cysteine (gamma glutamylcysteine) which can also be transported into cells. So, it is obvious that a
product high in the non-denatured conformation of these three proteins is essential for increasing intracellular glutathione levels.
Side Note:
Glutathione is one of
Dr. Clarkís favorite detox supplements because
Glutathione helps the body maintain good health despite occasional low-level benzene exposure.

Glutathione -
$47.95
30 Day Supply
We take great care in manufacturing products that do NOT contain excipients, like flow agents, coatings, colorings, binders, and release agents that
other manufacturers list under
"Other Ingredients", such as Silica, Stearate, Maltodextrin, Tocopherols, Derivatives from soy, and more.
For this reason our capsules may not have a uniform look and may not be completely full. Please rest assured that your capsule weight is as stated on the label, we pay close and systematic attention to weight, purity and the potency of our capsules. Our number one goal is PURITY and POTENCY, not LOOKS.
It takes an abundance of patience and care to encapsulate powders without flow agents. As a result, we often run our machines at 10% of full speed in order to get the job done. Again
PURITY is our #1 goal, not saving on production COST.
No one else takes the time to make such PURE products.
|
-
Ames, B. N., Shigenaga, M. K. & Hagen, T. M. (1993). Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 90: 7915-7922.
-
Baruchel, S, Viav, G., Olivier. R., Bounous, G., Wainberg, M. (1996). Nutraceutical modulation of GSH with a humanized native milk serum protein isolate, Immunocal: Application AIDS and cancer is oxidative stress and redox regulation: Cellular signaling, AIDS, cancer and other diseases. Symposium, Institute Pasteur
-
Bjorck, L., Rosen, C., Marshall, V., and Reiter B. (1975). Antibacterial Activity of the Lactoperoxidase System in Milk Against Pseudomonads and Other Gram-Negative Bacteria, Appl. Microbiol., 30, 199.
-
Bounous, G., and P. Gold. (1991). The biological activity of un-denatured whey proteins: role of glutathione. Clin. Invest Med.; 14:296-309
-
DeLeve LD, Kaplowitz N. (1991). Glutathione metabolism and its role in hepatotoxicity. Pharmacol Ther Dec; 52(3):287-305. Review.
-
Droege, W., E. Holm. (1997). Role of Cysteine and glutathione in HIV infection and other diseases associated with muscle wasting and immunological dysfunction. [Review] [91 refs.]. FASEBJ 1997, 11:1077-1089.
-
Droege, W., HP Eck, S. Mihm, D. Galter. (1994). Abnormal redox regulations in HIV infection and other immunodeficiency diseases. In: Oxidative Stress, cell activation and viral infection. C. Pasquier et al. (eds..). Birkauser Verlag, Basel, Switzerland, 285
-
Gutman, J. Schettini, S. (1998). The ultimate GSH handbook. Montreal: Gutman and Schettini Enr.
-
Harmsen, M.C., et al. (1995). Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J. Infec. Dis. 172, 380-388.
-
Kawakami, H. et al. (1993). Effect of lactoferrin on iron solubility under neutral conditions. Biosci. Biotech. Biochem. 57(8), 1376-1377.
-
Kawasaki et al. (1993). Inhibition of kappa-casein glycomacropeptide and lactoferrin of influenza virus hemagglutination. Biosci Biotech Biochem 57 (7), 1214-1215.
-
Lomaestro BM, Malone M. (1995). Glutathione in health and disease: pharmacotherapeutic issues. Annals Pharmacotherapy; 29:1263-73
-
Marchetti et al. (1998). Metal complexes of bovine lactoferrin inhibit in vitro replication of Herpes simplex virus type 1 and 2. BioMetals 11, 89-94.
-
Marx, J.J.M., and van Asbeck, B.S. (1996). Use of iron chelators in preventing hydroxyl radical damage: Adult Respiratory Distress Syndrome as an experimental model for the pathophysiology and treatment of oxygen-radical-mediated tissue damage. Acta Hematology. 95, 49-62.
-
Meister, A. (1984). New aspects of glutathione biochemistry and transport selective alteration of glutathione metabolism. Nutr Rev 42: 397-410
-
Meister, A. (1994). The antioxidant effects of glutathione ascorbic acid. In: Oxidative stress, Cell Activation and Viral Infection. C. Pasquier et al. (eds..) Birkauser Verlag Basel, Switzerland 101-111
-
Noelle, RJ, DA Lawrence. (1981). Determination of glutathione in lymphocytes and possible association of redox state and proliferative capacity of lymphocytes. Biochem. J. 198:571-579
-
Reiter, B. (1985). The biological significance of the non-immunoglobulin protective proteins in milk. Developments in Dairy Chemistry, 3, 281-336.
-
Renner, E. (Ed.), (1989). Micronutrients in milk and milk-based food products. 1-70, 134-138, 275-276.
-
Samaranayake et al. (1997). The antifungal effect of lactoferrin and lysozyme on Candida krusei and Candida albicans, APMIS 105, 875-883.
-
Sanchez L., Calvo M., Brock, J.H. (1992).
TMBiological role of lactoferrinTM, Archives of Disease in Childhood, 67, 657-661.
-
Superti et al. (1997). Antirotaviral activity of milk proteins: lactoferrin prevents rotavirus infection in the enterocyte-like cell line HT-29, Med Microbiol Immunol 186, 83-91.
-
Yamauchi, K. Biologically functional proteins of milk and peptides derived from milk proteins, 75th Annual Session of the IDF, Tokyo, Oct. 1991.
-
Yi, M. et al. (1997).
Hepatitis C virus envelope proteins bind lactoferrin. J. Virol. 71 (8), 5997-6002.
.png)

Glutathione
|







